Client: Apellis
Role: Head of Product, Squint Metrics
Effort: 6 months
Launched: pending
A full-scale remote natural history study application for patient’s with certain anemic conditions. The Fitbit enabled application serves as a multi-faceted data collection system, complex reporting to study participants on disease effect on activity and fatigue, an adherence engine for monitoring study compliance, and study reimbursement for adherence using TangoCard. The application also works as a recruitment tool for phase 3 clinical trials. Admins can control all access and invites while keeping subject data anonymous.
Program Screens (tablet)
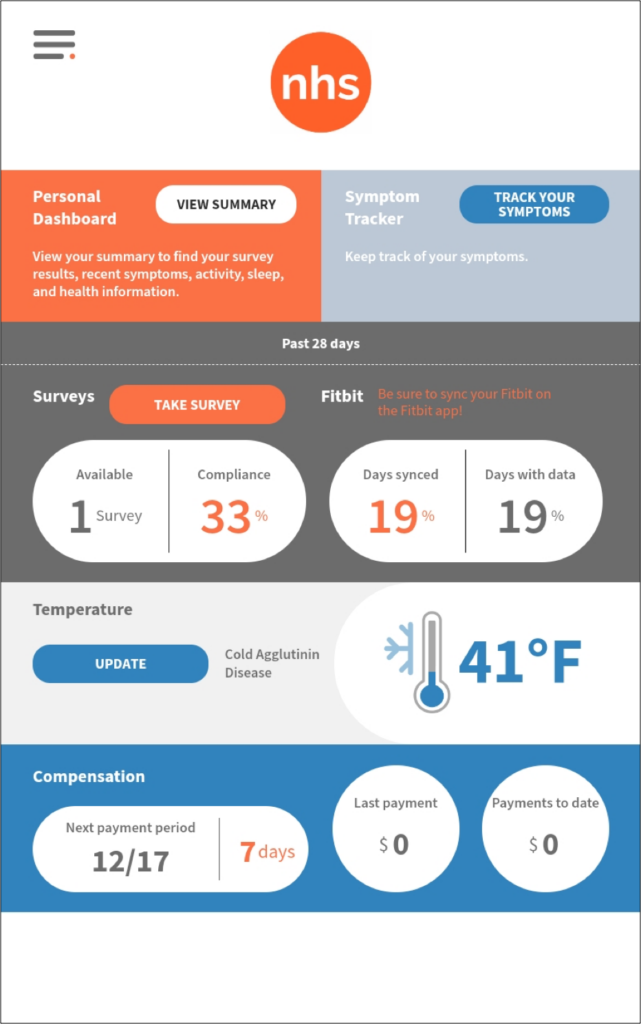
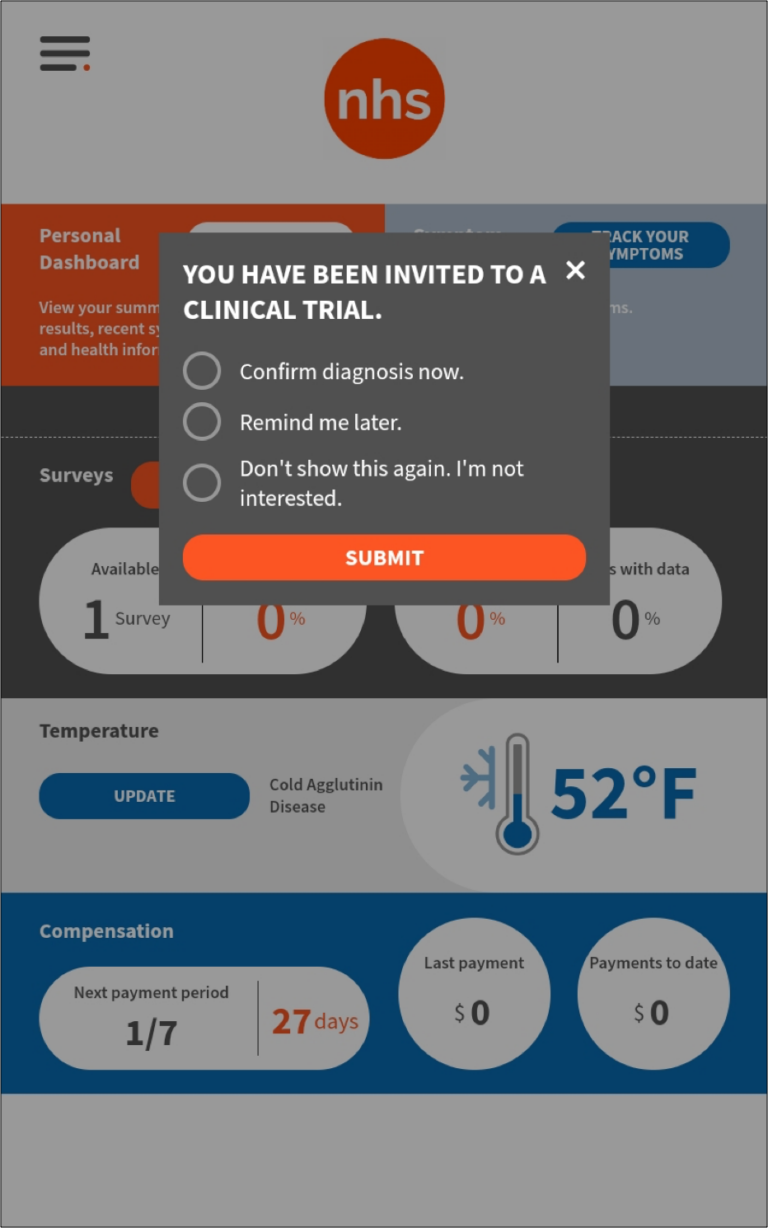
Subject Dashboard
The homepage of the subject tablet offers all information required to stay compliant, get reimbursed, and monitor all aspects of the NHS study.
50% of monthly surveys and 50% of the days need to have Fitbit data synced to the system in order to ensure compliance and receive the monthly $50 compensation.
In-app reporting
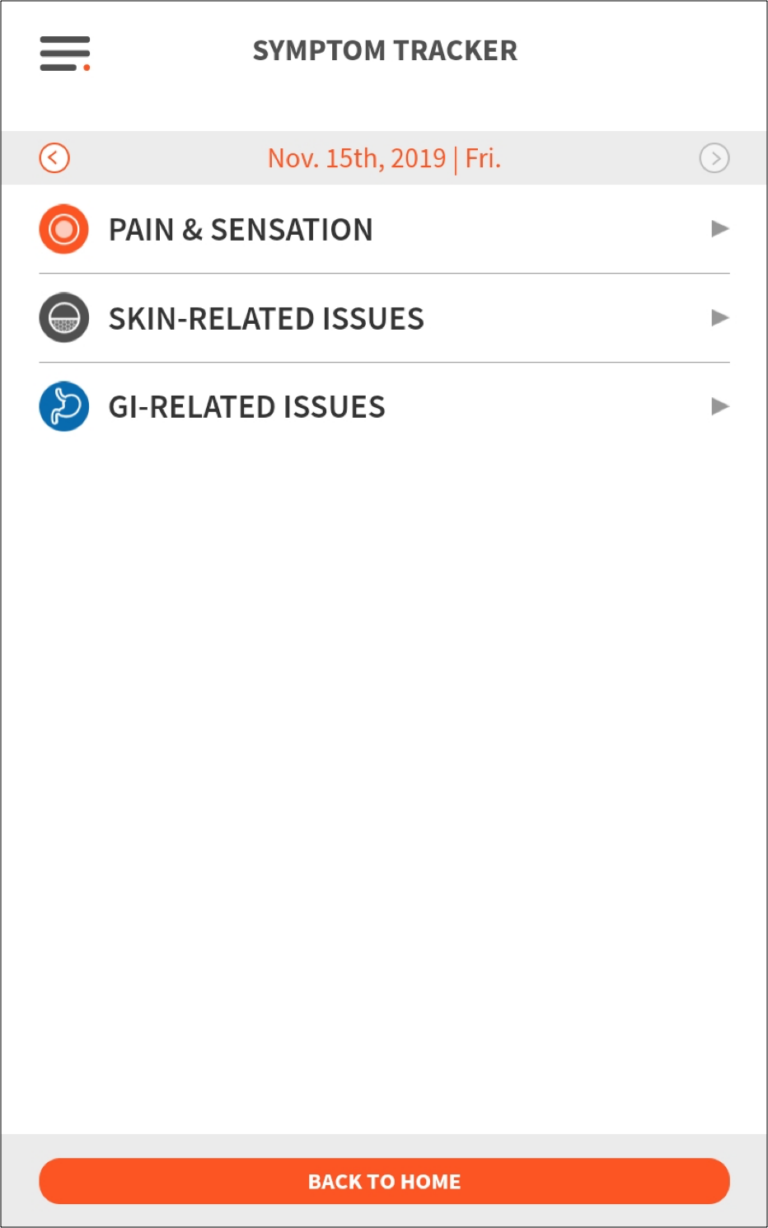
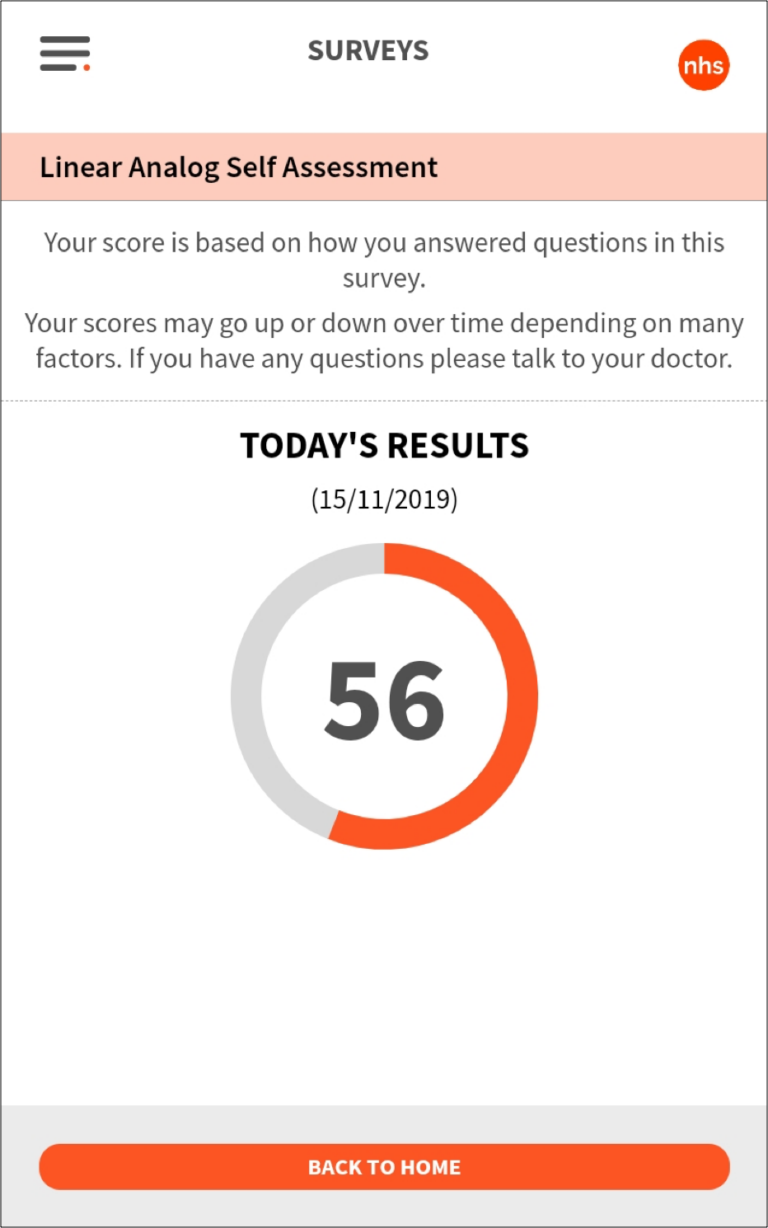
The subject reporting dashboard aggregates all of the study information from the Fitbit, symptom reporting, and surveys and plots it all together within a date range to reveal insights about the relationship between disease symptoms and quality of life.
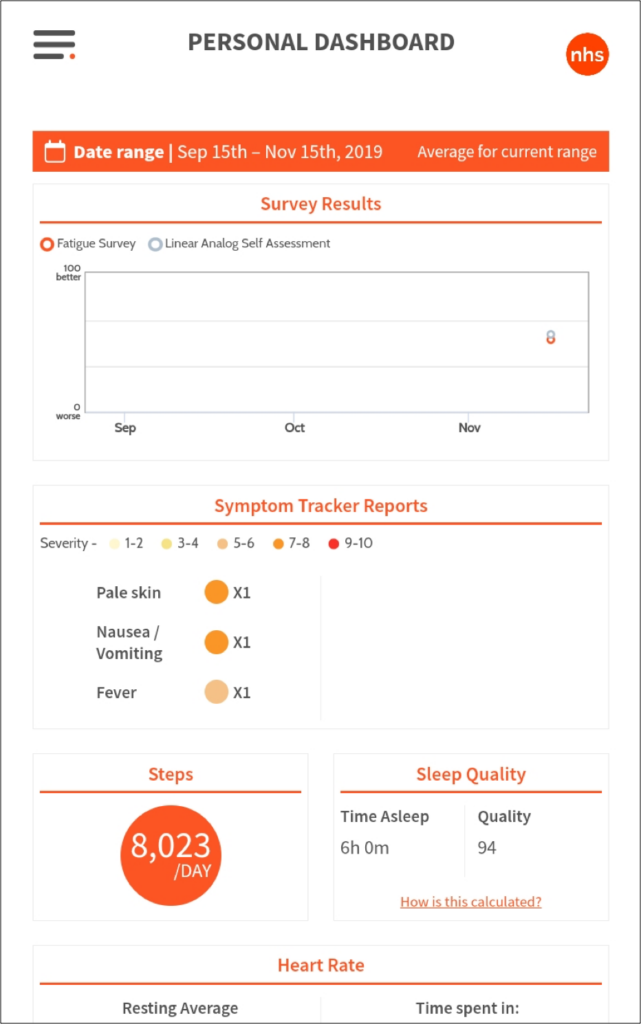
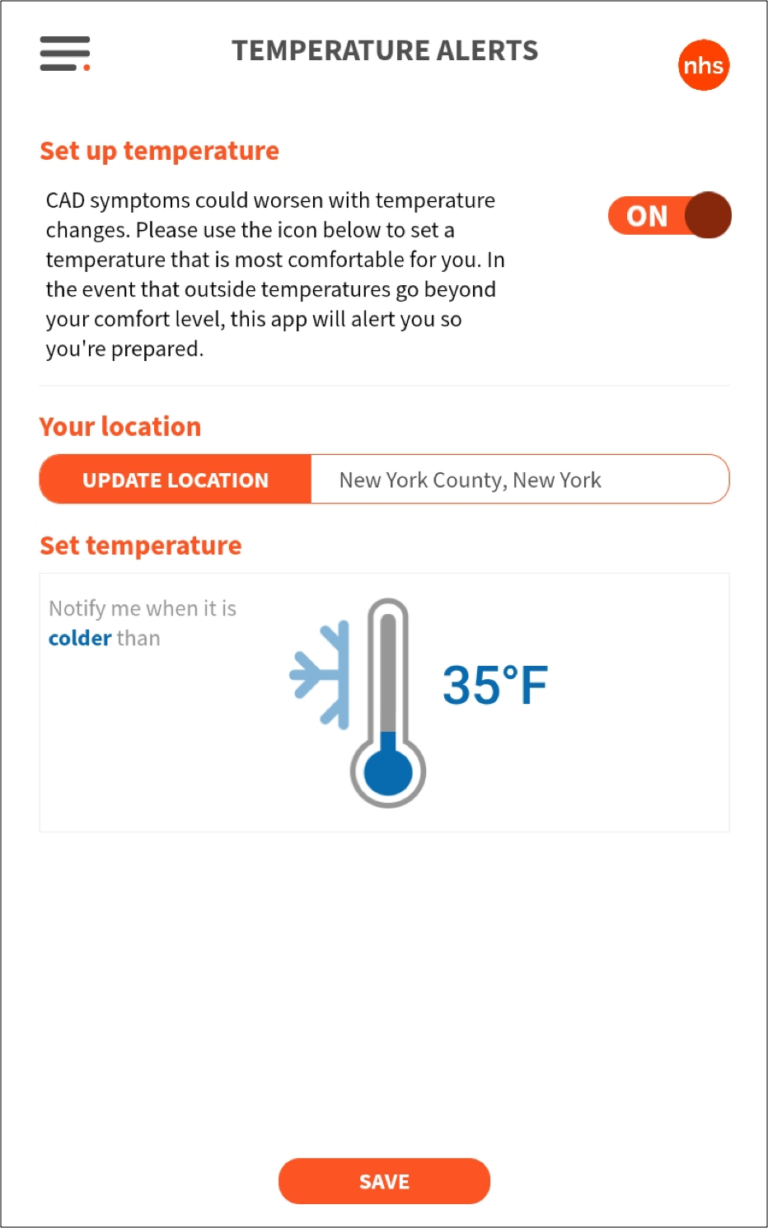
Weather alerts are used to warn of warm and cold sensitivities due to anemic conditions (some life-threatening). In addition to the app there is a complex CRM system for status notification as well as 3 tiers of admin dashboards.